cr full electron configuration|2.6: Electron Configurations : Cebu After the 4s is full we put the remaining six electrons in the 3d orbital and end with . Ferdie Candelaria, senior coordinator of the Mandaluyong City Medical Center Vaccination, urged all businesses in the city to register their employees and essential workers with the city's vaccination program, MANDAVAX, to ensure that all workers in the city can be protected against COVID-19.
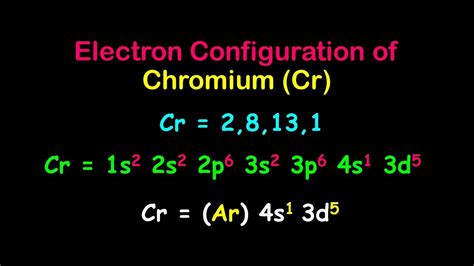
cr full electron configuration,In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six .

After the 4s is full we put the remaining six electrons in the 3d orbital and end with .cr full electron configuration 2.6: Electron Configurations How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .

In order to write the Mg electron configuration we first need to know the .We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since .Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
March 23, 2023. Electron configuration chart of all Elements . The answer is [Ar] 4s1 3d5Because half-filled subshells are somewhere stable, the atom prefers to have a half-filled 4s and half-filled 3d's than a full 4s a. © 2024 Google LLC. To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number.All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. The electron configurations and orbital diagrams of these four elements are: .Chromium is a chemical element with atomic number 24 and represented by the symbol Cr in the Periodic Table. Chromium is a lustrous, hard metal that has a silver-grey colour. It .Watch on. Chromium, with its atomic symbol Cr, has an electron configuration of [Ar] 3d^5 4s^1. This means that there are a total of 24 electrons in the chromium atom, with .
Chromium electron configuration in an easy language is basically the process in which the chemical element distributes its electrons in a given system of .The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals have different sublevels, thus can hold more electrons. As stated, the . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number . To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number. In a similar manner, the 3s orbital will have 2 electrons and then 3p will assimilate the 6 further electrons. for the 4s orbital, we will have the 2 electrons and then the remaining electrons will be held by the 3d orbitals. In the end, this whole distribution process will what make the Cr electron configuration 1s22s22p63s23p44s23d9.
Electron configurations are written using the principal quantum number n, followed by the orbital (s, p, d, or f) with the total number of electrons written as a superscript. Example: 1s 2 For writing ground state electron configurations, a few main steps should be followed. Find the amount of electrons in the atom. Example: Na: 11 e .Cr is an exception where the last electron enters into the 3d orbital instead of 4s orbital to attain half-filled stability. The electronic configuration of chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5. Cr 3 + is formed by losing three electrons from the neutral chromium atom. So, the electronic configuration of Cr 3 + is 1 s 2 2 s 2 2 .Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation .
cr full electron configuration Cr and Cu are the two exceptions of electron configuration of atoms up to Kr. this is because a 1/2 or completely full D block has extra stability, therefore in the case of Chromium . The answer is [Ar] 4s1 3d5Because half-filled subshells are somewhere stable, the atom prefers to have a half-filled 4s and half-filled 3d's than a full 4s a.
Gallium has 31 electrons so the full electronic configuration is: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p1 [Ar] 3d 10 4s 2 4p 1. Even though the 4s is filled first, the full electron configuration is often written in numerical order. So, if there are electrons in the 3d sub-shell, then these will be written before the 4s . Cr is [Ar] 3d 5 4s 1 .
Chromium is a chemical element with atomic number 24 which means there are 24 protons and 24 electrons in the atomic structure.The chemical symbol for Chromium is Cr. Electron Configuration and Oxidation States of Chromium. Electron configuration of Chromium is [Ar] 3d5 4s1. Possible oxidation states are +2,3,6. .The electron configurations of silicon (14 electrons), phosphorus . (shown in Figure 6.29) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among others, are not those we would expect. In general, such exceptions involve subshells with very similar energy, and small effects can lead to changes in the .
Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 52 Cr Electron configuration [Ar] 3d 5 4s 1 CAS number: 7440-47-3 ChemSpider ID:Chromium is a chemical element of the periodic table with chemical symbol Cr and atomic number 24 with an atomic weight of 51.9962 u and is classed as a transition metal. . Full configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1: Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 5: 4s 1: Electrons per shell: 2, 8, 13, 1: Valence .2.6: Electron Configurations Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .
Half filled orbitals for "Cr" in particular is its most stable configuration. So the electron configuration for elemental Chromium is 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(1)3d^(5). And the electrons in the 4s orbital is removed first because this orbital lies further from the nucleus, making electrons easier to .
Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. It looks something like this.The electronic configuration of chromium is 1s2 2s2 2p6 3s2 3p6 4s1 3d5. The first five subshells contain the core electrons of chromium and can be condensed to the symbol for argon. The valence electrons of chromium are found in the 4s and 3d .
cr full electron configuration|2.6: Electron Configurations
PH0 · Electron Configuration of Cr (Chromium)
PH1 · Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules)
PH2 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH3 · Electron Configuration for Chromium (Cr and Cr2+, Cr3+ ions)
PH4 · Electron Configuration For Cr
PH5 · Electron Configuration Chart of All Elements (Full Chart)
PH6 · Chromium Electron Configuration (Cr) with Orbital Diagram
PH7 · Chromium (Cr)
PH8 · 2.6: Electron Configurations
PH9 · 2.4 Electron Configurations